TOKYO, Oct 16, 2024 - (JCN Newswire) - Eisai Co., Ltd. announced today that amyotrophic lateral sclerosis (ALS) treatment “Rozebalamin® for Injection 25 mg” (mecobalamin) has received the Good Design Award 2024 (by the Japan Institute of Design Promotion) for its light-proof vial packaging.
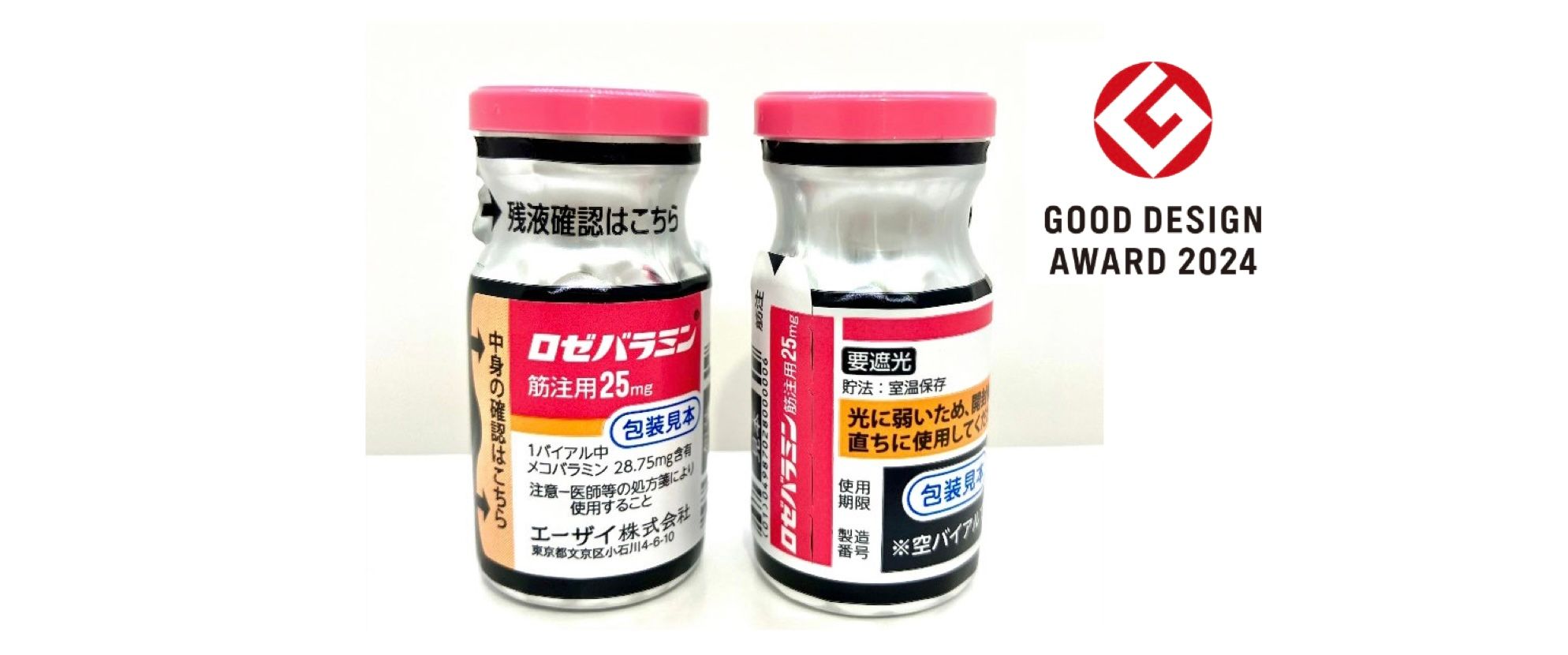
“Rozebalamin for Injection” was approved in Japan on September 24, 2024 as a treatment for amyotrophic lateral sclerosis. Its active ingredient is mecobalamin, which was developed as a freeze-dried formulation which is dissolved into a solvent at the time of administration. Mecobalamin is chemically unstable when exposed to light, therefore requiring a higher level of light-protection than that of the commonly used light- blocking film made by depositing aluminum onto a PET film (aluminium-deposited PET), from the time of distribution/storage until immediately before administration. However, there is also some necessity for visibility, such as the ability to visually confirm the medication inside the vial at the time of use and see the needle tip when extracting the medication with a syringe.
For Rozebalamin, packaging was created with a high level of light-protection by applying a black print to aluminium-deposited PET. While the entire vial is covered by the light-blocking packaging, a resealable window to visibility to inspect the solution inside, and the neck of the vial allows that can be peeled back. In addition, perforations were not added to the film with the aim of maintaining the high capacity for light- protection. In doing so, the conflicting issues of increasing light-protection while maintaining visibility were solved, which was also highly evaluated in this selection. This packaging was co-developed with IL Pharma Packaging Co., Ltd. (Headquarters: Aichi), and the award was also presented to both companies.
Eisai considers neurology a therapeutic area of focus. As a human healthcare company, Eisai is committed to further addressing the diverse needs of, and increasing the benefits of, patients, their families, and healthcare professionals, by providing high-quality and easy-to-use Rozebalamin as a new treatment option for ALS patients.
About Good Design Award
A Japanese design evaluation and promotion mechanism that was first established in 1957 as the Good Design Product Selection System. As a global design award with participation from numerous domestic and international companies and organizations, it is held annually with the aim of improving the quality of life and utilizing design to solve social issues and themes. The "G Mark," which symbolizes the award, is widely recognized as an emblem of excellent design. www.g-mark.org/en.
Media Inquiries:
Public Relations Department, Eisai Co., Ltd.
+81-(0)3-3817-5120